Machine Perfusion of the Pancreas: What Can We Learn from five Decades of Experimental Studies?
Mauricio Flores Carvalho1, Fabio Staderini2, Janina Eden3, Nadia Navari2, Mattia Dimitri 4, Andrea Corvi4, Fabio Cianchi2, Adriano Peris5, Paolo Muiesan6,7, Philipp Dutkowski3, Fabio Marra1,8, Daniele Dondossola7, Andrea Schlegel1,3,7,9*
1 Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy
2 Department of Surgery and Translational Medicine, University of Florence, Firenze, Italy
3 Swiss HPB and Transplant Center, Department of Visceral Surgery and Transplantation, University Hospital Zurich, 8091 Zurich, Switzerland
4 Department of Industrial Engineering, University of Florence, Firenze, Italy
5 Tuscany Regional Transplant Authority, Centro Regionale Allocazione Organi e Tessuti (CRAOT), Florence, Italy
6 Hepatobiliary Unit, Careggi University Hospital, University of Florence, 50134 Florence, Italy
7 Fondazione IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Centre of Preclinical Research, 20122 Milan, Italy
8 Center for Research, High Education and Transfer DENOThe, University of Florence, Florence, Italy
9 Department of Pathophysiology and Transplantation , Università degli Studi Milan, 20122, Italy
*Corresponding author: Andrea Schlegel, Fondazione IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Centre of Preclinical Research, 20122 Milan, Italy
Received Date: 18 March, 2023
Accepted Date: 21 March, 2023
Published Date: 23 March, 2023
Citation: Flores Carvalho M, Staderini F, Eden J, Navari N, Dimitri M, et al. (2023) Machine Perfusion of the Pancreas: What Can We Learn from five Decades of Experimental Studies?. J Surg 8: 1760 DOI: https://doi.org/10.29011/2575-9760.001760
Abstract
Background: Pancreas transplantation is currently the best option for patients with severe complications of diabetes. This organ is however particularly vulnerable to Ischemia-Reperfusion-Injury (IRI) and the transplant procedure is associated with a high risk for recipient complications. It is therefore surprising, that testing and routine use of dynamic preservation strategies is lacking behind other solid organs.
Methods: This study includes first, a literature review on the evolution of cold and warm pancreas machine perfusion strategies. Second, pressure-controlled hypothermic oxygenated perfusion (HOPE, pO 2>60kPa) with fluoresceine is performed in porcine pancreases.
Results: No single human pancreas transplant study with machine perfusion is available. A few older animal studies exist with prolonged Hypothermic Machine Perfusion (HMP), with however high perfusion pressures and a lack of active perfusate oxygenation. Tissue oedema and inflammation were the direct consequences. Only recently, such HMP-conditions were adapted, providing an actively oxygenated perfusate at lower perfusion pressures and shorter durations. Such HOPE- treatment was found superior to cold storage and normothermic perfusion in early experimental studies. In our series, HOPE achieved a complete pancreas perfusion, as confirmed by fluorescence despite lower perfusion pressures.
Conclusion: HMP with active perfusate oxygenation may achieve similar protective effects in pancreases as seen with livers and kidneys. Lower perfusion pressures appear sufficient to distribute the required oxygen for mitochondrial reprogramming to reduce posttransplant IRI. Prospective clinical studies are planned to test the HOPE-technique in human pancreas transplantation.
Keywords: Hypothermic oxygenated perfusion; Oxygen; Pancreas; Perfusion parameters; Perfusion quality; Mitochondria
Introduction
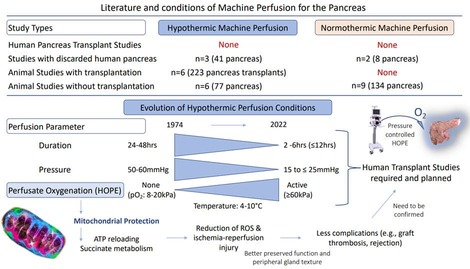
Pancreas transplantation is the most effective treatment for patients with diabetes and related severe complications, including end-stage renal disease [1-4]. The pancreas is however particularly vulnerable to ischemia-reperfusion-injury (IRI) with microcirculatory failure and severe complications, including graft thrombosis and pancreatitis, responsible for 20-50% of graft losses [2,5,6]. Graft pancreatitis is directedly related to advanced IRI and often clinically silent. One third of such cases occurs as an early form within 3 months after transplantation and leads to graft loss in up to 90% [6]. The Standard Cold Storage (SCS) appears therefore insufficient to preserve such glands, particularly when procured from marginal donors or after circulatory death (DCD) with a high donor risk profile. Based on this and with the increasing donor age, the risk-appetite is rather low and leads to an overall high global pancreas discard rate of 30-50% with the exception of a few experienced centres [1,5]. Dynamic preservation techniques have gained renewed attention in all solid organs. Despite the first application of this technology in pancreases as early as in 1974, the routine clinical use of such concepts lacks behind most other solid organs [7-9]. The first perfusion concept includes the recirculation of artificial perfusion fluids under cold conditions. Although this Hypothermic Machine Perfusion (HMP) technique was tested in pancreas as early as in kidneys, it is not used in clinical pancreas transplantation yet. This is in contrast to the field of kidney transplantation, where HMP techniques have achieved commissioning for routine clinical use in several countries[10]. With an increasing need to improve available organs, the HMP equipment is currently developed further and the role of real-time viability tests is explored. While the HMP concept was introduced first in the United States (US), centres in Europe have started to use this technology to perfuse high-risk human livers in 2012 using highly oxygenated perfusates. The protective effect of this Hypothermic Oxygenated Perfusion (HOPE) on posttransplant outcomes was described in many retrospective clinical studies and randomized controlled trials (RCTs) in various solid organs [10-13]. Available studies showed a reduction of early allograft dysfunction (EAD) and recipient complications (e.g., biliary complications for livers and acute rejection for kidneys), better graft survival and lower retransplantation rates after liver and kidney transplantation [10-12,14,15]. The underlying mechanism of the HOPE-technique is based on mitochondrial protection with the reestablishment of an aerobe cellular respiration, previously interrupted during warm and cold ischemia. The direct consequence is the metabolism of accumulated toxic metabolites (e.g., succinate, NADH) and the reloading of Adenosine-Trisphosphate (ATP). This was demonstrated in hearts, lungs, livers and kidneys with a strict dependency on high perfusate oxygen levels of >60kPa [16-20]. In addition, a HOPE-duration of 2hrs seems important to effectively recharge cells with enough ATP [21,22].
In contrast, in most studies hypothermic pancreas perfusion was prolonged with >24hrs to replace cold storage with high perfusion pressures between 30-60mmHg [9,23-29]. Only more recently, such conditions were challenged and modified with lower pressures and shorter perfusion durations. Surprisingly, the most relevant molecule, oxygen, was kept rather low without active perfusate oxygenation in most studies [9,23,24,26-28]. Such controversial perfusion conditions are thought to be one main reason for the slow progress with dynamic perfusion technologies in pancreas transplantation. With the recent improvement of available perfusion devices, Normothermic Machine Perfusion (NMP) of the pancreas is increasingly explored. The advantage is the opportunity to test viability and to “imitate” the transplant setting as preclinical intervention [7,30,31]. Comparative clinical studies with transplantation after cold or warn machine perfusion are lacking. Only a few experimental studies exist, but not only for pancreas, also with other solid organs, such as livers or kidneys [32-34]. Only two studies are currently ongoing comparing different preservation methods in liver transplantation. For the pancreas, the next step is to better understand the underlying mechanisms of such techniques and the potential clinical benefit. The aim of this study was therefore to first, critically review and discuss the current literature on different perfusion concepts for the pancreas and secondly, to provide an initial experience of the HOPE-technique in pancreases with a perfusion device, routinely used for clinical liver and kidney perfusions before transplantation.
Materials and Methods
Literature Review
To identify the most beneficial settings for our perfusion series, a literature review was performed first, summarizing the evolution of the literature of pancreas machine perfusion between 1974 and 2023. The entire spectrum of machine perfusion concepts was considered.
Porcine Model and Experimental Groups
Next, porcine pancreases were procured en-bloc with liver and the bowel package from adult pigs (slaughterhouse). Healthy control pancreases were immediately flushed with UW-solution and sampled as control (DBD baseline control group, n=6). Organs in the injury group underwent 90min asystolic donor warm ischemia time (DWIT), followed by cold flush and 5hrs standard cold storage (SCS; n=6). In the perfusion group, such pancreases with DWIT underwent additional 2hrs of HOPE (n=6). To assess the pancreas perfusion quality, additional HOPE-experiments were done with the substitution of fluoresceine (0.25g; concentration 0.5g/5ml) to the perfusate (n=3).
Hypothermic Oxygenated Perfusion (HOPE) of The Pancreas
At the end of 90min DWIT all pancreas were flushed and stored in UW-solution for 5hrs. During later bench preparation another flush with 500ml UW-solution was performed and organs were separated from the liver, the bowel and the spleen with meticulous closure of small vascular branches to ensure appropriate perfusion. Both arterial vessels, supra-mesenteric and splenic artery were cannulated and used as perfusion route. Portal- and splenic veins were kept untouched for a passive outflow. The VitasmartÒ (Bridge to life Ltd; Medica) was used for the 2-hour HOPE procedures with UW-Machine Perfusion Solution (UW-MPS). This pressure-controlled device is CE- marked for clinical liver and kidney perfusions. The circuit with the standard disposable available for clinical applications was used and includes pressure, flow and temperature sensors and provides an active perfusate oxygenation (Oxygenator: EurosetÒ EU5054). The device monitors pressure, flow, resistance and temperature in real-time.
Endpoints
Perfusate oxygen levels were measured through blood gas analysis. Further parameters of perfusion quality were measured by the device. HOPE-perfusates were obtained to quantify perfusate levels of Flavin-Mononucleotide-Levels (FMN) and NADH using spectroscopy. Such parameters are increasingly discussed as surrogate of mitochondrial function and injury [35-37]. Based on previous studies with other organs, perfusates were obtained within the first 30min of HOPE. Shortly, triplicates of 50ml perfusate were pipetted into standard 96 vial plates with a dilution of 1:4. Perfusate FMN levels were measured using the standard technology of spectroscopy with an excitation and emission wavelength of 485nm and 528nm, respectively. The perfusate NADH-levels were determined with an excitation and emission wavelength of 360nm and 460nm, respectively. Further details regarding the methodology can be found elsewhere [16,38,39]. At the end of cold storage or additional HOPE the pancreas weight was measured and tissues were obtained for histological assessment. Standard processing with formalin and embedding procedures were performed. Three staining procedures were done: Hematoxylin-Eosin (H&E), Toll-like-receptor-4 (TLR-4; macrophages, dendritic cells, Lifespan Bioscience: LS-B2070) and von Willebrand factor (vWF; endothelial cells, DAKO: A0082). Quantifications of TLR-4 - positive and vWF-positive cells were determined by manual counting in 20 random visual fields per experimental group. All histological analyses were performed in a blinded fashion with respect to the experimental groups. Vessels were excluded from the analysis.
Statistics, Quality Control and Ethical Approval
Completeness, plausibility, and validity of the data were independently verified (by MFC, RP and AS). Continuous variables are demonstrated as median and interquartile range (IQR). Statistical analysis was performed using the non-parametric Mann- Whitney-Wilcoxon U-test (GraphPad Prism, version 7.0, San Diego, CA, USA). P-values of <0.05 were considered significant. The experiments were carried out according to European Union (EU) directive guidelines (2010/63/EU) and Italian legislation (DLgs 26/2014) at the Centro Polyvalent Florence University (Cubo; Viale Gaetano Pieraccini, 6, 50139 Firenze FI, Italy).
Results
What Is Available In The Literature?
In 1974, Eloy et al presented the first study with Normothermic Machine Perfusion (NMP) of a canine pancreas, performed for 100min to test the secretory pancreas function [8]. This was paralleled by a first experimental comparison of 24hrs hypothermic machine perfusion (HMP) and SCS with subsequent transplantation of the canine pancreas [9]. Although the outcome of this pioneer study was not very promising, it triggered a series of experimental studies in the following years and instigated a controversial discussion regarding the best possible perfusion concepts. A total of 23 studies, 15 with HMP and 11 with NMP, were reported in the literature. The majority included pancreases from brain death donors (DBD; n=18) exposed to long CS or HMP. While no study exists today with transplantation of perfused human pancreas, five studies utilized discarded human pancreases [29,40-43]. Only Hamaoui et al have assessed IRI-features during NMP after previous HMP or standard CS in discarded human pancreases. In this study, HMP was performed with active oxygenation (HOPE) and resulted in a better organ functionality (Table 1)[41]. Dogs (n=8) and pigs (n=9) were the most frequent species in non-human studies. Since the seventies, the following perfusion systems were referenced in the literature as being used for pancreas HMP including the GambroÒ, the RM3 Machine Perfusion Unit, the Max-100 perfusion device (Waters Medical SystemÒ), the Waves kidney perfusion device or LifePort Ò kidney transporter [5,26,27,29,41].
Normothermic Pancreas Perfusion
The known advantage of Normothermic Machine Perfusion (NMP) as seen in other solid organs is the opportunity to assess viability. To perform the best possible NMP with near-physiologic conditions, an optimized equipment is needed. Organ positioning is one key feature to avoid pressure necroses particularly during prolonged perfusion as recently described with full and partial human livers [44,45]. The first experimental pancreas study with NMP was done as early as in 1974 [8]. Until today, two studies describe NMP in discarded human pancreas (Table 1) and nine experimental non-transplant studies were published with pancreases from pigs, dogs or rodents (Supplementary Table 2). Unfortunately, the studies with human organs lack a comparator or baseline control group [42,43]. Barlow et al performed a short (1- 2hrs) endischemic NMP of discarded DBD pancreases after 13hrs cold storage and demonstrated the link between a higher donor risk and perfusate amylase and lipase levels. This study also showed higher levels of insulin secretion from younger pancreas[42]. Three of five organs developed focal acinar necrosis and one showed extensive fat necrosis in histology. In contrast, Nassar et al performed NMP for 6hrs after shorter cold storage of only 4hrs with better results. Such human pancreas showed healthier acini at the end of 6hrs NMP with normal chromogranin staining (Table 1)[43]. Such findings parallel recent clinical studies with NMP in livers. Posttransplant results were superior with NMP when performed instead of cold storage compared to an endischemic approach [46]. NMP in the recipient center after transport led to a comparably high rate of non-anastomotic strictures in livers with higher risk, e.g., from DCD donors [47,48]. Animal studies further supported the recent findings with perfusion of human pancreases. Shorter endischemic NMP of 90min to 5hrs resulted in well-maintained tissue and limited inflammation[8,30,31,49-54]. Most studies included DBD pancreas and lack a high risk donor model. In-house devices and ECMO-based equipment were used by many. Various perfusate compositions were reported in the literature with autologous whole blood or washed erythrocytes as oxygen carriers in most recent studies [30,31,49]. Particularly in older studies the cold ischemia time before NMP is frequently not reported (Supplementary Table 2) [8,52-55].
What are Optimal Perfusion Conditions for Hypothermic Machine Perfusion?
Five transplant studies explored the effect of non-actively oxygenated, prolonged HMP (24-48hrs) with high perfusion pressures (30-60mmHg) [9,23,24,56]. Three studies showed an equal or even better recipient survival and pancreas function after 24-48hrs SCS compared to such high-pressure-HMP [9,23,24]. Already in 1975, Tersigni et al described improved posttransplant results with lower perfusion pressures of 10-25mmHg[25]. While a perfusion at too low pressures (e.g., 10mmHg) led to incomplete pancreas perfusion with limited protective effects, high pressures between 30 and 70 mmHg caused pancreatic edema [5,7,24,27]. Such findings were paralleled by Karcz et al, who showed good outcomes and a minimal weight gain with a pressure between 15- 23mmHg [57]. Mild or moderate edema may be even beneficial for islet digestion and isolation [49]. Most authors would probably agree with a peak perfusion pressure of 15-25mmHg during HMP [1,5,7]. Next, in addition to organ temperature and tissue quality, perfusion flow is related to the set pressures. Toledo-Pereyra et al demonstrated superiority of a pulsatile flow for arterial systems, to maintain sheer-stress regulating the inflammatory response of endothelial cells; an enhanced expression of Kruppel-like- factor-2 is discussed as relevant for the microcirculation with potential antithrombotic properties [5,58]. Flow- and pressure related effects are also linked to the perfusion duration. Most older studies aimed to replace SCS with HMP and the subsequently long HMP-duration contributed to pancreas edema with often no superiority compared to SCS, particularly with high pressures. Shorter HMP, e.g., 12-24hrs, was found superior compared to 48hrs HMP, where posttransplant survivals were seen inferior as demonstrated by Florack et al [5,23,58]. The addition of mannitol to the perfusate can help to minimize graft edema [1,41,57]. In 1992, Kenmochi et al explored the impact of a short 1hr endischemic HMP demonstrating more pancreas edema, inflammation and dysfunction with prolonged Donor Warm Ischemia Times (DWIT) of 30-60min [56]. Despite this important step towards an endischemic HMP-approach, the perfusion was probably too short, with high pressures of 50mmHg (Table 1)[56]. Such findings were paralleled by perfusion studies in other organs. Results from HOPE-treatment of livers demonstrated that too high pressures induce damage already during the first perfusion hour and diminish the protective effect achieved with high perfusate oxygen levels [22].
How Much Perfusate Oxygen is Beneficial During Hypothermic Perfusion?
In recent studies, perfusion pressure and duration were reduced to 25mmHg and 6hrs, respectively. The low perfusate oxygen levels may be an additional reason for the limited HMP- effect in earlier studies. Authors often claim to use an oxygenated perfusate with however no further details on oxygenator type or size and the lack of perfusate oxygen partial pressures (Table 1). Only two recent studies present perfusate oxygen levels [41,59]. Lemkuil et al from the UK have demonstrated the perfusate oxygen-dependent ATP-reloading of the pancreas, similar as seen with other solid organs. Authors describe less inflammation and better endo- and exocrine pancreas function during later evaluation on the normothermic perfusion device (Tables 2,3)[40]. Similar results are known from kidneys and livers; perfusate oxygen levels of 21% or a pO2 of <20kPa (8-18kPa) is not enough to trigger metabolic changes and rebuild ATP [60-64]. Subsequently, HMP without active oxygenation as done with the LifePort for many years, has limited effects. A recent RCT demonstrated clear superiority of oxygenated HMP in kidney transplantation [6]. Similar findings are seen with livers; deoxygenated HOPE induced the same IRI as seen with unperfused, cold stored controls [22,65]. Such results are paralleled by the observed tissue protection through the simple addition of oxygen to cold stored organs with persufflation (bubbling) techniques. Higher tissue ATP levels were seen after oxygen persufflation through the graft vasculature. Similarly, as with above described oxygenated HMP such pancreases demonstrated lower levels of IRI and less tissue edema after normothermic reperfusion [66]. The high relevance of oxygen was also demonstrated with the development of preservation concepts where oxygen can be added to standard cold storage [66]. Kuroda et al. from Japan presented in 1988 a new “Two-layer Method”. Perfluorocarbon was added to the conventional cold storage flush solution [67]. These molecules have the unique ability to reversibly bind much more oxygen with a 20-fold higher oxygen concentration compared to human blood. As shown in experiments, Perfluorocarbon forms the lowest layer below the cold storage solution and the oxygen diffuses passively into the pancreas with subsequent ATP recovery. A few early experimental studies demonstrated a prolonged viability of organs. An experimental transplant study showed that slightly more recipients of two-layer method preserved pancreases achieved insulin independency compared to standard cold storage [68]. Although these studies confirm the high relevance of oxygen, the effective penetration of oxygen into deep tissue layers without perfusion remains unproven. Of interest appears also another perfusion parameter: the perfusion duration. Several authors advocate for a 2hrs cold perfusion to achieve the required metabolic switch of mitochondria [16,21,22]. Within the first minutes of HOPE, oxygen reactivates mitochondrial complex proteins with normally directed electron flow and metabolism of previously accumulated toxic metabolites, (e.g., succinate and NADH). Such metabolic changes lead to lower ROS-levels and are key protective effects, also described with HOPE in livers, hearts and kidneys [16,18,19,22,69]. The ATP-reloading during HOPE in pancreas as demonstrated by Leemkuil et al parallels such findings [40].
|
Year, author, country |
Number and type of Pancreas |
Experimental groups Perfusion mode |
Cold ischemia time before perfusion |
Perfusion Duration |
Perfusate oxygenation |
Perfusion settings |
Perfusion device & solution |
Results |
Discussion |
|
Experimental Studies with hypothermic perfusion of discarded human pancreas and with evaluation during normothermic reperfusion |
|||||||||
|
Hamaoui et al, J of Surg Research, 2018 |
Porcine DCD and declined Human (30- 55min dWIT) with HMP vs. SCS, 3 study phases (n=12 overall) |
Hypothermic oxygenated, endischemic (after SCS), with 2hrs assessment during NMP (phase 2&3) |
3-7hrs, 24hrs (porcine); 26.8 and 56hrs (human) |
5hrs |
Active: Yes pO : 0.95-2.8 kPa2/min/mL/g |
Temperature: 4°C Pressure: 26-37mmHg (phase 1: 30mmHg, phase 2&3: 20mmHg) Flow: 24.8-29.7mL/ min/100 g; PFI: 0.7-1.19 mL/min/100 g/ mmHg |
Waters Medical RM3 Machine Perfusion Unit , modified UW solution |
Better endocrine viability and pancreas functionality with HMP-O . Weight gain between 3.9-140% in diffe 2rent groups during HMP, low pressure led to very limited weight changes |
No transplant, but evaluation during NMP, no information on oxygen levels in perfusate. |
|
Experimental Studies with hypothermic perfusion of discarded human pancreas without evaluation during normothermic reperfusion |
|||||||||
|
Branchereau J et al, Cryobiology 2018, |
Human, DBD, discarded, HMP (n=7) vs. SCS (n=2; 12 & 24hrs), split group: p-head HMP, body/tail SCS |
Hypothermic, pulsatile instead of cold storage |
Not available* |
24hrs |
Active: Yes (low) pO : Not ava2ilable |
Temperature: 4°C Syst. Pressure: 25mmHg Flow: pulsatile, rate not available |
Ò Waves Ò Belzer (Perf Gen ), Belzer machine perfusion solution |
Cold stored pancreases developed ischemic lesions, which were not seen with HMP, normal immunhistochemistry, RI: 0.22- 0.25, Insulin, glucagon normal after HMP |
No transplant or evaluation during NMP, no information of cold storage duration before HMP, no information on oxygen levels in perfusate. |
|
Leemkuil et al, Transplantation direct, 2018, |
Human, DBD or DCD (14-24min dWIT) with HOPE or SCS (5 each group, total 20) |
Hypothermic oxygenated, endischemic (after SCS) |
6hrs |
6hrs |
Active: Yes (flow: 100ml/ min) pO : Not ava2ilable |
Temperature: 4-7°C Syst. Pressure: fixed at 25mmHg Flow: median 36ml/min (DCD), 38.5 and 52ml/min (DBD) |
Modified device from Ò Organ Assist , UW machine perfusion solution |
HMP-O uploaded ATP (6.8 fold DCDs, 2.6 fold 2DBD), high islet viability after isolation from DCD pancreas after HMP-O , no signs of ROS-release or inflamm2ation; Amylase, Lipase, LDH increased after HMP-O 2, |
No transplant or evaluation during NMP, active oxygenation, no information on oxygen levels in perfusate. |
|
Experimental Studies with nomothermic perfusion of discarded human pancreas without transplantation |
|||||||||
|
Barlow AD et al, AJT 2015, UK |
Human, DBD, discarded, endischemic NMP (n=5) |
NMP |
13hrs |
1-2hrs |
Active: Yes, physiological, pO : Not ava2 ilable |
Temperature: 37°C Syst. Pressure: fixed at 50- 55mmHg Flow: mean: 35 ± 2.8mL/ min/100g |
ECMO (pediatric pump, cardiopulmonary bypass), blood based + gelofusine, with sodium, hepatine, mannitol, glucose |
Higher perfusate amylase and lipase with higher donor risk, more insulin secretion in pancreas from younger donors, 3/5 with focal acinar cell necrosis, 1/5 with extensive parenchymal and fat necrosis |
No transplantation, small case number, DBD model, no comparator group, short perfusion |
|
Nassar A et al, Artificial Organs, 2018, USA |
Human, DBD, discarded, endischemic NMP (n=3) |
NMP |
4hrs 6min |
6hrs (n=2), 12hrs (n=1) |
Active: Yes, physiological, pO : Not ava2 ilable |
Temperature: 37°C Syst. Pressure: fixed at 60mmHg Flow: mean: 55mL /min/100g |
In-house device, RBC and plasma, 1:3 ratio |
C-peptide levels increased up to 18ng/ml within 6hrs NMP, healthy looking acini at 6 (n=2) and 12hrs (n=1), chromogranin staining normal |
No transplantation, small case number, DBD model, no comparator group |
*: considering that human pancreas are flushed cold and procured there should be some time of SCS, also because the procurement was en-bloc with donor livers, requiring back table separation and vascular reconstruction for the two main arteries; Fixed pressures is a pressure which is kept at a certain level by the perfusion device and subsequently maintains a specific flow; organ cannot regulate how much flow it prefers, but is forced to accept a specific pressure and flow.
Table 1: Literature overview on hypothermic and normothermic machine perfusion of the human pancreas.
|
Year, author, country |
Number and type of Pancreas |
Experimental groups |
Cold ischemiatime preperfusion |
Perfusion Duration |
Perfusate oxygenation |
Perfusion settings |
Perfusion device & solution |
Results |
Discussion |
|
Experimental studies with hypothermic perfusion of animal pancreas with Transplantation |
|||||||||
|
Brynger H, Eur Surg Res, 1975, Sweden |
DBD, mongrel dog pancreas, recipient pancreatectomy 2days before transplantation, n=20 donors and recipients |
24hrs HMP (n=7), vs. 24hrs SCS (n=9), direct transplantation (n=4) |
HMP or SCS, no relevant SCS before HMP |
24hrs |
Active: yes, no oxygenator, oxygenation of organ chamber pO : Not ava2ilable |
Temp.: 6-8°C Pressure: 50/36- 44mmHg Flow: 95ml/min |
Gambro perfusion machine with oxygenation, buffer-inverted sugar solution |
Significant edema during HMP (weight gain 130-270%), HMP: 4/7 death due to bleeding (n=3/7), hypoglycemia (n=1), SCS: 4/9 death due to bleeding (n=3/9), volvolus (n=1/9), |
Loss of recipients due to bleeding in the two main groups, no information on oxygen levels |
|
Tersigni R et al, Ann Surg, 1975, USA |
DBD mongrel dog pancreas, n=25, allotransplantation |
Healthy controls (n=5), HMP 24hrs: at 5mmHg (n=5), at 10mmHg (n=5), at 10mmHg with MCPP (n=5), at 25mmHg (n=5) |
24hrs HMP, no relevant SCS before HMP |
24hrs |
Active: yes (2l/ min) pO : Not ava2ilable |
Temp.: 6°C Pressure: 5, 10 or 25mmHg Flow: pulsatile, 10- 60ml/min based on pressure |
Pulsatile, max 100, Waters, CPP solution (methylpred, KCl, mannitol, penicillin, MgSO MCPP 4), |
Better recipient survival with 10mmHg perfusion pressure with MCPP solution instead of HMP with CPP or perfusion with higher pressure of 25mmHg, better enzyme secretion |
Experimental group with 25mmHg and MCPP solution is missing, no information on oxygen levels |
|
De Gruyl et al, Br J Surg, 1977, Netherlands |
DBD dog pancreas, n=38, 19 transplants |
healthy control (n=9), 24hrs HMP (n=5), 24hrs SCS (n=5) |
HMP or SCS, no relevant SCS before HMP |
24hrs |
Active: yes pO : Not ava2ilable |
Temp.: 6-10°C Pressure: 60mmHg Flow: not available |
laboratory Belzer machine, CPP solution |
Better survival with 24hrs HMP compared to SCS, lower insulin peak in HMP and SCS compared to healhy controls |
High perfusion pressure, no information on oxygen levels |
|
Florack G et al, J of Surg Res, 1983, USA |
DBD, mongrel dog pancreas, pancreatectomy and segmental pancreas tail transplantation, n=98, 4 and 12 weeks follow up |
HMP: with SGF-I and II (24hrs, n=12 each; 48hrs, n=8-10 each); SCS: Collins solution (24hrs, n=12; 48hrs, n=10),or SGF-1 solution, 24hrs (n=12), 48hrs (n=12), 72hrs (n=10), healthy controls, transplant (n=20) |
HMP or SCS, no relevant SCS before HMP |
24-48hrs |
Active: yes pO : Not ava2ilable |
Temp.: 4°C Pressure: 30mmHg Flow: pulsatile, 4.5 (initially), 6.5 (24hrs), 6.3 (48hrs) ml/min SGF-1, 5.3- 8ml/min SGF-II |
Pulsatile perfusion, SGF-I (dextrose), SGF-II (no dextrose, but Mannitol, Insulin, PSP dye)* |
Lower peak amylasis with HMP, long-term pancreas function rate better with SCS compared to HMP (50% with 48hrs HMP with SGF-II vs. 75% with 48hrs SCS with SGF-I), severe edema after high pressure HMP |
Assessment of different perfusion solutions, long HMP, no informaiton on oxygen levels |
|
Kenmochi T. et al, Transplantation, 1992, Japan |
DCD, dog pancreas, transplants n=13, total n=26 |
DCD grafts with different donro warm ischemia times: 15min (n=5), 30min (n=9) or 60min (n=7), DBD (no ischemia) controls (n=4) |
Details not available, but direct perfusion |
1hr |
Active: yes pO : Not ava2ilable |
Temp.: 6-10°C Pressure: 50mmHg Flow: 69.3ml / min/100g |
ORPH3000C perfusion machine, CPP solution, fibrinogen-free plasma |
Groups with 30 & 60min DWIT had edema, more weight gain in 60min DWIT group, decreasing amylasis with higher DWIT, graft prognosis did not correlate with DWIT |
High perfusion pressure, no comparison with longer perfusion >1hr, no information on oxygen levels |
|
Prudhomme et al, Transplant international, 2021, UK & France |
Porcine pancreas, Feasibility ex- situ perfusion of pancreas, second part: allotransplantation: n=14 |
Feasibility, HMP vs SCS (n=3 each), transplantation with SCS or HMP of 2 or 6hrs (n=14), diabetic porcine recipient |
HMP or SCS, no relevant SCS before HMP |
24hrs, 2hrs, 6hrs |
Active: no oxygenator, 21% O , flow: 1l/min 2 pO : >21kPa (1520mmHg) |
Temp.: 4-7°C Syst. Pressure: 15mmHg Flow: pulsatile, rate not available |
Waves© machine, Perfusate: IGL-1 |
Safety and feasibility to transplant in a porcine diabetes model, no differences in recipient survival comparing HMP and SCS, mean survival: 14 days |
Pancreas with low risk, perfusate not actively oxygenated |
MCPP: (replace mannitol, and add albumin, dextrose); HMP-O2: hypothermic perfusion with active oxygenation; PFI: perfusion flow indices; RI: resistance index; SCS: standard cold storage.
Table 2: Literature overview on Hypothermic Perfusion of animal pancreas with subsequent transplantation.
|
Year, author, country |
Number and type of Pancreas |
Experimentalgroups Perfusion mode |
Cold ischemia time before perfusion |
Perfusion Duration |
Perfusate oxygenation |
Perfusion settings |
Perfusiondevice & solution |
Results |
Discussion |
|
Experimental studies with animal pancreas WITHOUT transplantation but with evaluation during normothermic reperfusion on a device |
|||||||||
|
Ogbemudia et al, Transplant international, 2021, UK & France |
Porcine DCD pancreas, 15-30min DWIT |
SCS (n=4), HMP-O with UW (n=4) or with IG2 L-2 (n=5) |
3hrs SCS (HMP-O group), 2 9hrs SCS group |
6hrs |
Active: Yes (low), casette oxygenator, 21% O , flow: 1l/min 2 pO : >21kPa (1520mmHg) |
Temp.: 4-7°C Syst. Pressure: 15mmHg Flow: pulsatile, rate not available |
Waves machine with UW MPS or IGL-2 |
Higher flow rates and lower resistance No during NMP after HMP compared to SCS, du tissue weight decreased in HMP group pe compared to SCS, edema/patchy ischemia HM during NMP of SCS pancreas, homogenous 21 NMP after HMP |
transplant, but evaluation ring NMP, improved rfusion conditions during P, active oxygen but only kpa |
|
Experimental studies with hypothermic perfusion of animal pancreas WITHOUT Transplantation or evaluation during normothermic reperfusion on a device |
|||||||||
|
Karcz M et al, Exp Clin Transplant, 2010, UK |
Porcine pancreas, DCD (25min total DWIT), n=15 |
Only one group with HMP |
150min |
315min |
Active: Yes pO : Not available 2 |
Temp.: 4-10°C Pressure: 5-13mmHg (first 60min), then 15- 23mmHg Flow: >65ml/min/g |
Waters Medical RM3 Machine Perfusion Unit, Belzer MPS |
Pancreas weight gain 3.2-18.3g, Significant reduction of acinar cell and islet cell damage during HMP |
No transplant or evaluation during NMP, only one experimental group, no information on oxygen levels |
|
Taylor MJ et al, Cell transplant, 2010, USA |
Porcine pancreas, DBD and DCD |
Healthy control (n=7), 24hrs SCS with or without prior DWIT (n=9 each), HMP with KPS-1 or Unisol with or without prior 30min DWIT (n=7 each) |
0hrs (HMP), max 2hrs control |
24hrs |
Active: No pO : Not available 2 |
Temp.: 5-7°C Pressure: 10mmHg, Flow: n.a. |
Lifeport kidney transporter, Unisol or KPS-1 |
Islet isolation for viability testing, higher viable islet yield after HMP, also after DWIT, moderate edema |
No transplant study or evaluation during NMP, no information on oxygen levels |
|
Weegman BP et al, Cell Transplantation, 2012 |
Porcine pancreas perfusion and islet isolation and transplantation (nude mice) |
HMP (n=4) vs SCS (n=6) |
24hrs |
Active: No pO : Not available 2 |
Temp.: 4-8°C Pressure: 10mmHg, Flow: n.a. |
Lifeport kidney transporter, Unisol or KPS-1 |
Islets isolated and transplanted, 4/4 islet recipients after HMP had complete diabetes reversal compared to 5/6 after SCS |
Small case load, no information on oxygen levels, no whole organ transplantation or evaluation during nmp |
|
|
Prudhomme et al, Artificial organs 2020 |
Baboon pancreas, SCS control (n=2), HMP (n=5) |
SCS vs HMP: 3 groups, perfusion pressure 15mmHg (n=3), 20mmHg (n=1), or 25mmHg (n=1). |
minimal |
24hrs |
Active: Yes pO : Not available 2 |
Temp.: 4°C Pressure: 15, 20, 25mmHg Flow: pulsatile, rate not available |
Waves kidney perfusion device, IGL-1 |
Similar edema with different perfusion pressures, increasing injury with prolonged SCS or HMP of >12hrs. |
No transplant or evaluation during NMP, small numbers in groups with different perfusion pressures |
HMP-O2: hypothermic perfusion with active oxygenation; PFI: perfusion flow indices; RI: resistance index; SCS: standard cold storage.
Table 3: Literature Review on Hypothermic Perfusion of the animal pancreas without subsequent transplantation.
|
Author,year, country |
Number and type of Pancreas |
ExperimentalgroupsPerfusion mode |
Cold ischemia before perfusion |
Perfusion Duration |
Perfusate oxygenation |
Perfusion settings |
Perfusiondevice&solution |
Results |
Discussion |
|
Eloy et al, Eur Surg Res, 1974, France |
DBD dog pancreas, n=15 |
NMP only, test endo-+exocrine hormone response |
Not available |
100min |
Active: Yes pO2: Not available, O2 -consumption: 0.75mL/min/100g |
Temperature: 39-40°C Pressure: not available Flow: 38-46mL/ min/100g |
Roller pump, disc oxygenator, heat exchanger, momologous erythrocytes+Earles salt, glucose, albumine, dextrane |
Glucose tolerance test: constant insuline secretion, increased when glucose reached 140mg/mL, excocrine response to secretin/ cholecystokinin infusion |
No transplantation, DBD model, no comparator group, short perfusion, |
|
Loubatières- Mariani MM et al, Diabetologia 1980, France |
DBD model of wistar rats pancreas, n=unspecified |
Low or high glucose concentration to stimulate secretion at 28°C or 37.5°C, affect of Tolbutamide, Acethylcholine, Arginine assessed |
Not available |
90min |
Active: Yes pO2: not available |
Temperature: 28 or 37.5°C Syst. Pressure: 35cmH2O (37.5°C group); 37.5cmH2O (28°C) Flow: 2.4ml/min |
Unspecified perfusion system, perfusion with glucose for 45min + Krebs Ringer bicarb buffer+albumin, glucose |
Lower insulin output at lower temperatures; Glucagon secretion not different at 28 and 37.5°C; Insulin response to Acethylcholine was less at 28 °C than at 37.5°C; B cell response to tolbutamine was less at 28°C; Glucagon secretion stimulated by Arginine |
Short NMP, no histological assessment, no transplantation |
|
Eckhauser F et al, J Surg Res, 1981, USA |
DBD dog pancreas, n not available |
NMP group (n not available) |
Not available |
4-5hrs |
Active: Yes pO2: Not available |
Temperature: 37°C Pressure: 90- 110mmHg initially, 30-50mmHg Flow: 15-24mL/min |
Perfusion apparatus, pulsatile, water bath heating,in- line dialysis, autolog red cells+albumin, mannitol, heparin |
Edema in all pancreas, vascular resistance and oxygen consumption suggested as viability criteria, |
No transplantation, unknown case number, no comparison with other techniques or perfusion conditions, cold storage prior to NMP not available |
|
Pegg DE et al, J Surg Res, 1982, UK |
DBD model, WAG rats, n=45 |
Recirculation (n=5) for 2hrs; Single-pass method for 2hrs (n=40), |
Not available |
2hrs |
Active: Yes pO2: not available |
Temperature: 38°C Syst. Pressure: 60mmHg Flow: 2ml/min |
Single pass method (recirculation of perfusate) or two way tap, Gelatine polypeptide Haemaccel+calcium,+/- glucose |
Lower wet/dry weight ratio with single pass method compared to perfusate recirculation |
No transplantation |
|
O`Maller VP et al, J Surg Res 1986, USA |
DBD model of dog pancreas, n=24 |
Healthy controls (n=6), pancreatitis group (n=6), fluosol-perfused controls (n=6), fluosol-perfused pancreas with pancreatitis (n=6) |
Not available |
4hrs |
Active: Yes pO2: Not available |
Temperature: 37°C Syst. Pressure: 61- 95mmHg Flow: 20ml/min |
In-house perfusion device, heated chamber, oxygenator, sensors; Fluosol (FC- 43)+Glucose, albumin or blood based with autologous blood+heparin, albumin, glucose |
Pancreatitis grafts have edema and with fluosol the edema is reduced, lower Amylase-release with fluosol compared to blood-based NMP, histology comparable |
No transplantation, panceatitis model instead of no donor model comparable to clinical situation, cold storage not reported, |
|
Wahlberg J et al, Transplant Int 1989, USA |
DBD model of dog pancreas, n=30 |
Healthy control (n=6); 24 or 48hrs SCS + NMP with/without Allopurinol (n=6 each; 4x6); |
24 and 48hrs |
2hrs |
Active: Yes pO2:350-450 mmHg |
Temperature: 37°C Syst. Pressure: 61- 95mmHg Flow: 20ml/min |
In-house device, roller pump, heat exchanger, oxygenator; UW solution with dextran with/without allopurinol |
No effect of allopurinol, more weight gain and amylase release during NMP in SCS pancreas |
No transplantation, NMP with UW and short |
|
Kuan KG et al, Artif. Organs, 2017 UK, Australia |
DBD porcine pancreas, n=4 |
Isolated pancreas perfusion (n=2), pancreas and kidney (n=2) |
34min |
2-4hrs |
Active: Yes pO2: Not available |
Temperature: 37°C Pressure: 70-80mmHg Flow: 0.37L/min |
Roller pump system, water bath heating, autolog whole blood-based |
Moderate/severe edema after 90min NMP, acinar damage, inflammation and thrombosis after NMP |
No transplantation, small case number, no comparison with other techniques |
|
Kumar R et al, In J Surg, 2018, UK |
Porcine pancreas (n=9) |
Control pancreas (50mmHg pressure, 3h perfusion) vs 20mmHg & 4hrs perfusion; |
127 and 136min |
4hrs |
Active: Yes mean arterial O2 pressures: 76.7kPa; venous: 6.2kPa |
Temperature: 37°C Syst. Pressure: 50mmHg à 141ml/ min flow; 20mmHg à 40ml/min flow |
In-house device, autologous blood, heparin, cefuroxime |
Low pressure (20mmHg) pancreas NMP achieved better results with significnatly better cell death profiles (e.g. lower anti- caspase 3 positivity and ATP-synthetase activity compared to 50mmHg |
No transplantation, minimal injury |
|
Parmentier C et al, JOVE 2022, Canada |
Porcine DBD pancreas, n=7 |
NMP, one group, n=7 |
2hrs |
3hrs |
Active: Yes pO2: Not available, Oxygen consumption 100- 200ml/min/g |
Temperature: 37°C Syst. Pressure: fixed at 20-25mmHg Flow: mean: 100- 120ml/min |
neonatal cardiopulmonary bypass equipment, washed erythrocytes, steen solution, ringers, heparin, bicarb, aprotinin |
NMP: pH: 7.35-7.4; Amylase increases to >20000U/L; Lipase to 3500 U/L; good histological results after 3hrs NMP |
No transplantation, small case number, DBD model, no comparator group |
*: considering that human pancreas are flushed cold and procured there should be some time of SCS, also because the procurement was en-bloc with donor livers, requiring back table separation; Fixed pressures is a pressure which is kept at a certain level by the perfusion device and subsequently maintains a specific flow; organ cannot regulate how much flow it prefers, but is forced to accept a specific pressure and flow; HMP-O2: hypothermic perfusion with active oxygenation; PFI: perfusion flow index; RI: resistance index; SCS: standard cold storage
Table 4: Overview on Normothermic Perfusion of animal pancreas.
Series of Experimental HOPE-Perfusions of The DCD Pancreas
DCD pancreas underwent 5hrs SCS and subsequent 2hrs HOPE treatment (n=6) with the following conditions: pressure controlled system with a perfusion pressure of 15-20mmHg; the perfusion flow was pulsatile and ranged between 80-110ml/min/g; the observed resistance index was 0.7-0.8; the measured perfusion temperature was 6-10°C; perfusate: 1.5l UW-Machine perfusion solution. The median pancreas weight was 324g (IQR: 52g) with a weight gain of 4.2-14.8% during perfusion. Perfusate oxygenation was kept above a pO2 of 60kPa, confirmed with blood gas analysis; pO2 pressures of 69-83kPa were achieved within the first 30min of HOPE and maintained thereafter. Three DCD pancreases underwent HOPE with fluoresceine and demonstrated a homogenous fluoresceine staining throughout the entire gland within the first 15min of perfusion. The histological assessment confirmed the fluoresceine distribution (Figure 1).
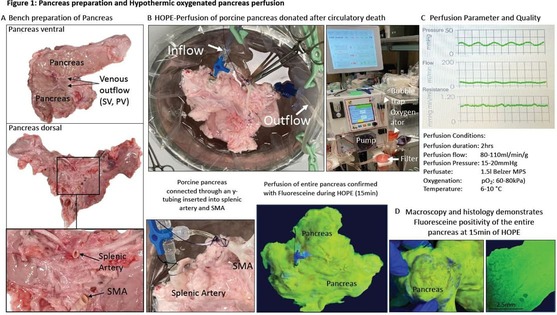
Figure 1: Pancreas preparation and Hypothermic oxygenated pancreas perfusion. Bench preparation of the porcine DCD pancreas (A) and cannulation of both arterial inflow vessels, e.g., SMA and SA to connect the pancreas with the device for hypothermic oxygenated perfusion (HOPE)(B). Pancreas perfusion parameters obtained during HOPE (C). Fluoresceine stained pancreas after 15min of HOPE, macroscopic and histological image after fluoresceine staining (D). HOPE: Hypothermic oxygenated perfusion; PV: portal vein; SA: splenic artery; SMA: supra-mesenteric artery; SV: splenic vein;
As described with different solid organs and first in hearts in 1969, mitochondrial complex-I releases flavin-mononucleotide (FMN) and NADH during re-oxygenation [16,22,39,69,70]. Such molecules have auto-fluorescent characteristics and are increasingly used for liver viability testing before transplantation. Expectedly, both molecules were released from pancreas mitochondria during HOPE with median perfusate concentrations of: FMN: 5461 A.U. (IQR: 4481-5926) and NADH: 7867 (IQR: 7066-8319) A.U..Compared to HOPE in livers and kidneys, the amount of released FMN in pancreases appears 10- and 3-fold lower, respectively, also based on the smaller organ size, the different mitochondrial density and metabolic activity of the pancreas.
The histological assessment demonstrated no signs of ischemic necrosis or congestion, minimal macrophage activation and inflammation at the end of 2hrs HOPE compared to low risk, baseline control DBD organs (Figure 2). The known dilatation of the peripheral vasculature was also seen here after HOPE of the pancreas (Figure 2A). This phenomenon was described earlier in other organs, e.g., livers or kidneys. With the need to completely preserve the entire periphery of this specific gland to reduce IRI-associated inflammation, this finding is of particular interest also in context of the here applied lower perfusion pressure of maximal 20mmHg during HOPE. No significant differences were observed regarding the number of proinflammatory tissue macrophages and endothelial cells throughout the study groups (Figure 2D).
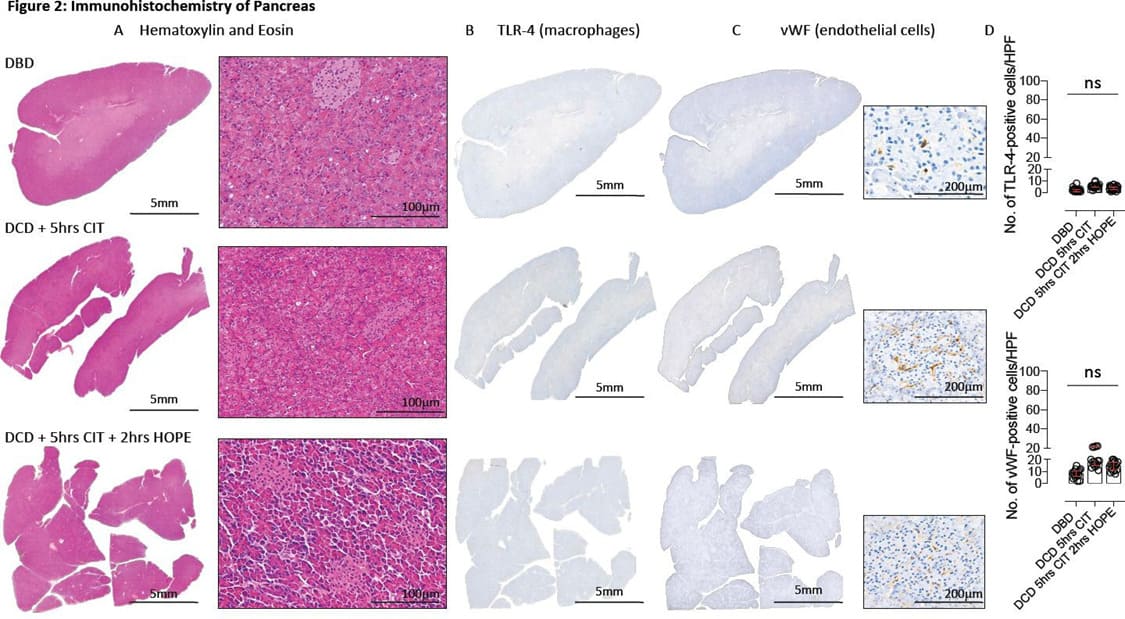
Figure 2: Immunohistochemistry of Pancreas. Images obtained after pancreas staining for Hematoxylin & Eosin (A), TLR-4 (B) and vWF-positive tissue (C). The number of positive cells were quantified per HPF (D); CIT: cold ischemia time; DBD: Donation after brain death; DCD: donation after circulatory death; HOPE: Hypothermic oxygenated perfusion; TLR-4: toll-like receptor 4; vWF: von Willebrand factor;
Discussion
Despite the early testing in 1974, pancreas perfusion remains in its infancy and the routine clinical implementation lacks behind other abdominal organs [71]. Only a few research programs exist to overcome described hurdles, which include the quality of existing studies (e.g., small case load with various, suboptimal perfusion conditions; a few, old studies with pancreas transplantation), the lack of human transplant studies and the small number of active pancreas transplant programs [5,71]. With the low vascularization, the pancreas is particularly sensitive to barotrauma, caused by prolonged perfusion and high pressures. Knowing the various benefits of dynamic organ preservation, including re-oxygenation, toxin-elimination, viability assessment, therapeutic interventions and endothelial cell protection, the pancreas should benefit most [41,72,73].
This literature review and cases series of HOPE in pancreas confirms what is reported with other organs. HMP should be performed at limited systolic pressures of £ 25mmHg for at least 2hrs, but ideally not more than 6-12hrs [5,7,74]. Once the oxygen has converted mitochondrial metabolisms and rebuilt energy in the entire gland, the perfusion pressure could potentially be reduced in cases, where longer perfusion is needed to bridge logistical challenges and to avoid relevant edema and necrosis. Such findings were further paralleled by a greater islet viability isolated from discarded human pancreas after HOPE as shown by Doppenberg et al in 2021[74]. More studies are required to identify such timings and to explore the acceptable SCS-duration before and after HOPE-treatment. The increasing interest in Normothermic Machine Perfusion (NMP) for all solid organs refers to the opportunity to quantify released IRI-associated molecules for viability assessment, which is particularly interesting in steatotic pancreas and organs from extended criteria donors. With regard to the preservation quality, comparative studies demonstrated however superiority of hypothermic perfusion techniques, thus none included a model of pancreas transplantation [5]. Hamaoui et al reported higher porcine and human pancreas damage with more tissue lesions after normothermic perfusion compared to HOPE [41]. Similar results were shown for livers. In addition, the injury observed during NMP was more pronounced with relevant SCS before perfusion. Recent clinical transplant studies showed this for livers, paralleled by two recent experimental studies with NMP in human pancreas [47,48,75,76]. While, Barlow et al demonstrated severe pancreas damage after endischemic NMP with previous 13hrs SCS, Nassar et al found healthy acini at the end of NMP performed after only 4-6hrs SCS, in a however small number of perfusions (n=3)[42,43]. As with HMP, perfusion pressures during NMP should not exceed 20mmHg[49]. A few groups with increasing expertise in pancreas perfusion are currently developing better circuits to further explore the role of NMP in pancreas. Barlow et al. demonstrated the feasibility of ex-situ pancreas NMP, where perfusate amylase levels correlated well with pancreas fat infiltration and exocrine function [5].
Most viability parameters assessed during NMP appear however quite peripheral to the known IRI-instigator, mitochondria. Pancreas injury and secretory function is frequently assessed during NMP through perfusate amylase and lipase levels; lactates, lactate dehydrogenase, insulin, glucagon, glucose, and C-peptide are further markers of injury and B-cell function [5]. The histology and the perfusion resistance indices may serve as additional parameters to evaluate the pancreas.
In contrast to such markers of downstream injury and function, molecules released from complex-I, such as FMN, were identified by many during the reperfusion of kidneys, livers, hearts and even brain under various conditions, and seem also evident in pancreas as shown here [38,39,70,77]. Perfusate FMN-levels are currently used to accept high-risk DCD livers for transplantation [10,50,52].
The experimental part of this study has some limitations. We are aware that this study does not present functional, molecular or dynamic parameters, which will require larger, prospective trials comparing most recent perfusion concepts in the same donor and recipient risk categories, as currently under preparation.
To increase the utilization of DCD pancreas, where in addition to exocrine dysfunction a low islet yield and beta cell function is known, organs would benefit from HOPE treatment and assessment, explored in upcoming studies [78-80].
The best perfusion conditions suggested for the pancreas based on current literature include: a pulsatile, hypothermic perfusion at 6-10 °C with pressure between 15-25mmHg and
related flows for a duration of 2-6hrs (max. 12hrs), using a highly oxygenated (pO2 >60kPa) perfusion solution (e.g., UW-MPS). Devices in current clinical use for livers and kidneys before transplantation can provide safe pancreas perfusion, given they are pressure controlled and administer the required perfusate oxygen needed to recondition mitochondria.
Author Contributions
Data curation, Fabio Staderini, Janina Eden, Nadia Navari and Andrea Schlegel; Formal analysis, Mauricio Flores Carvalho; Funding acquisition, Paolo Muiesan and Andrea Schlegel; Methodology, Mauricio Flores Carvalho and Andrea Schlegel; Resources, Mattia Dimitri, Fabio Cianchi, Adriano Peris, Paolo Muiesan, Philipp Dutkowski, Fabio Marra and Andrea Schlegel; Supervision, Andrea Schlegel; Writing - original draft, Mauricio Flores Carvalho and Andrea Schlegel; Writing - review & editing, Andrea Corvi, Philipp Dutkowski and Fabio Marra.
Funding
Funding was provided by the Swiss National Science Foundation grant no: 32003B-140776/1, 3200B-153012/1, 320030-189055/1, and 31IC30-166909 to P.D. and A.S. This study was supported by University Careggi grant no 32003B-140776/1 dedicated to P.M. This work was further supported by the OTT grant No.: DRGT641/2019 (cod.prog. 19CT03). The authors confirm that all funders played no role in study design, data collection, data analysis, interpretation, or writing of the report.
References
- Hamaoui K, Papalois V (2019) Machine Perfusion and the Pancreas:Will It Increase the Donor Pool? Curr Diab Rep 19.
- Gruessner A.C, Gruessner R.W.G (2016) Long-Term Outcome afterPancreas Transplantation: A Registry Analysis. Curr Opin OrganTransplant 21: 377-385.
- Boggi U, Vistoli F, Marchetti P, Kandaswamy R, Berney T, AndresA, Arbogast H.P, Badet L, Baronti W, Bartlett S.T (2021) First WorldConsensus Conference on Pancreas Transplantation: Part I-Methodsand Results of Literature Search. Am J Transplant 21: 1-16.
- Boggi U, Vistoli F, Andres A, Arbogast H.P, Badet L, Baronti W,Bartlett S.T, Benedetti E, Branchereau J, Burke G.W (2021) FirstWorld Consensus Conference on Pancreas Transplantation: Part II – American Journal of Transplantation 21: 17-p59.
- Prudhomme T, Kervella D, le Bas-Bernardet S, Cantarovich D, KaramG, Blancho G, Branchereau J (2020) Ex Situ Perfusion of Pancreas forWhole-Organ Transplantation: Is It Safe and Feasible? A Systematic J Diabetes Sci Technol 14: 120-134.
- Nadalin S, Girotti P, Königsrainer A (2013) Risk Factors for andManagement of Graft Pancreatitis. Curr Opin Organ Transplant 18:89-96.
- Branchereau J, Hunter J, Friend P, Ploeg R (2020) PancreasPreservation: Clinical Practice and Future Developments. Curr OpinOrgan Transplant 25: 329-335.
- Eloy R, Kachelhoffer J, Pousse A, Dauchel J, Grenier J.F (1974) ExVivo Vascular Perfusion of the Isolated Canine Pancreas. ExperimentalProcedure Haemodynamic Data and Experimental Applications. EurSurg Res 6 : 341-353.
- Brynger H (1975) Twenty-Four-Hour Preservation of the Duct-LigatedCanine Pancreatic Allograft. Eur Surg Res 7 : 341–354.
- Jochmans I, Brat A, Davies L, Hofker H.S, van de Leemkolk F.E.M,Leuvenink H.G.D, Knight S.R, Pirenne J, Ploeg R.J, Abramowicz D(2020) xygenated versus Standard Cold Perfusion Preservation inKidney Transplantation (COMPARE): A Randomised Double-BlindPaired Phase 3 Trial. The Lancet 396 :1653-1662.
- Schlegel A, Mueller M, Muller X, Eden J, Panconesi R, von FeltenS, Steigmiller K, Sousa Da Silva R.X, de Rougemont O, Mabrut J.-Y(2023) A Multicenter Randomized-Controlled Trial of HypothermicOxygenated Perfusion (HOPE) for Human Liver Grafts before J Hepatol.
- van Rijn R, Schurink I, de Vries Y, van den Berg A, Cortes CerisueloM, Darwish M, Erdmann J, Gilbo N, de Haas R, Heaton N (2021)Hypothermic Machine Perfusion in Liver Transplantation — ARandomized Trial. New England Journal of Medicine .
- Patrono D, Cussa D, Sciannameo V, Montanari E, Panconesi R,Berchialla P, Lepore M, Gambella A, Rizza G, Catalano G (2022)Outcome of Liver Transplantation with Grafts from Brain-Dead DonorsTreated with Dual Hypothermic Oxygenated Machine Perfusion withParticular Reference to Elderly Donors. Am J Transplant 22: 1382-
- Ravaioli M, Germinario G, Dajti G, Sessa M, Vasuri F, SiniscalchiA, Morelli M.C, Serenari M, del Gaudio M, Zanfi C (2022)Hypothermic Oxygenated Perfusion in Extended Criteria Donor LiverTransplantation-A Randomized Clinical Trial. Am J Transplant .
- Czigany Z, Pratschke J, Froněk J, Guba M, Schöning W, RaptisD, Andrassy J, Kramer M, Strnad P, Tolba R (2021) HypothermicOxygenated Machine Perfusion (HOPE) Reduces Early AllograftInjury and Improves Post-Transplant Outcomes in Extended CriteriaDonation (ECD) Liver Transplantation from Donation After Brain Death(DBD): Results from a Multicenter Randomized Con. Ann Surg .
- Schlegel A, Muller X, Mueller M, Stepanova A, Kron P, de RougemontO, Muiesan P, Clavien A, Galkin A, Meierhofer D (2020) HypothermicOxygenated Perfusion Protects from Mitochondrial Injury before LiverTransplantation. EBioMedicine 60.
- Kron P, Schlegel A, Mancina L, Clavien P.A, Dutkowski P (2018)Hypothermic Oxygenated Perfusion (HOPE) for Fatty Liver Grafts inRats and Humans. J Hepatol 68: 82-91.
- Wyss R, Méndez Carmona N, Arnold M, Segiser A, Mueller M,Dutkowski P, Carrel T, Longnus S (2020) Hypothermic OxygenatedPerfusion (HOPE) Provides Cardioprotection via Succinate OxidationPrior to Normothermic Perfusion in a Rat Model of Donation afterCirculatory Death (DCD). American Journal of Transplantation.
- Darius T, Vergauwen M, Smith T, Gerin I, Joris V, Mueller M, AydinS, Muller X, Schlegel A, Nath J (2020) Brief O 2 Uploading duringContinuous Hypothermic Machine Perfusion Is Simple yet EffectiveOxygenation Method to Improve Initial Kidney Function in a PorcineAutotransplant Model . American Journal of Transplantation.
- Nakajima D, Chen F, Okita K, Motoyama H, Hijiya K, Ohsumi A,Sakamoto J, Yamada T, Sato M, Aoyama A (2012) ReconditioningLungs Donated after Cardiac Death Using Short-Term Hypothermic Machine Perfusion. Transplantation 94: 999-1004.
- Koetting M, Lüer B, Efferz P, Paul A, Minor T (2011) Optimal Timefor Hypothermic Reconditioning of Liver Grafts by Venous SystemicOxygen Persufflation in a Large Animal Model. Transplantation.
- Schlegel A, Rougemont de; Graf R, Clavien P.A, Dutkowski P (2013)Protective Mechanisms of End-Ischemic Cold Machine Perfusion inDCD Liver Grafts. J Hepatol 58: 278-286.
- Florack G, Sutherland E.R, Heil J, Squifflet J.P, Najarian J.S (1983)Preservation of Canine Segmental Pancreatic Autografts: ColdStorage versus Pulsatile Machine Perfusion. J Surg Res 34: 493-504.
- de Gruyl J, Westbroek L, MacDicken I, Ridderhof E, Verschoor L, vanStrik R (1977) Cryoprecipitated Plasma Perfusion Preservation andCold Storage Preservation of Duct-Ligated Pancreatic Allografts. Br JSurg 64: 490-493.
- Tersigni R, Toledo Pereyra L.H, Pinkham J, Najarian J.S (1975)Pancreaticoduodenal Preservation by Hypothermic Pulsatile Perfusionfor Twenty-Four Hours. Ann Surg 182: 743-748.
- Taylor M.J, Baicu S, Greene E, Vazquez A, Brassil J (2010) IsletIsolation from Juvenile Porcine Pancreas after 24-h HypothermicMachine Perfusion Preservation. Cell Transplant 19: 613-628.
- Weegman B.P, Taylor M.J, Baicu S.C, Scott W.E, Mueller K.R,Kitzmann J.D, Rizzari M.D, Papas K.K (2012) Hypothermic PerfusionPreservation of Pancreas for Islet Grafts: Validation Using a Split LobePorcine Model. Cell Med 2: 105-110.
- Prudhomme T, Renaudin K, lo Faro M.L, Cantarovich D, Kervella D,Minault D, Hervouet J, le Bas-Bernardet S, Karam G, Blancho G (2020)Ex Situ Hypothermic Perfusion of Nonhuman Primate Pancreas: AFeasibility Study. Artif Organs 44: 736-743.
- Branchereau J, Renaudin K, Kervella D, Bernadet S, Karam G,Blancho G, Cantarovich D (2018) Hypothermic Pulsatile Perfusion ofHuman Pancreas: Preliminary Technical Feasibility Study Based on Cryobiology 85: 56-62.
- Kuan G, Wee M.N, Chung W.Y, Kumar R, Mees S.T, DennisonA, Maddern G, Trochsler M (2017) A Study of NormothermicHemoperfusion of the Porcine Pancreas and Kidney. Artif Organs 41:490-495.
- Parmentier C, Ray S, Mazilescu L, Kawamura M, Noguchi Y,Nogueira E, Ganesh S, Arulratnam B, Kalimuthu S, Selzner M (2022)Normothermic Ex Vivo Pancreas Perfusion for the Preservation ofPancreas Allografts before Transplantation. J Vis Exp.
- Schlegel A, Kron P, Graf R, Dutkowski P, Clavien A. Warm vs (2014)Cold Perfusion Techniques to Rescue Rodent Liver Grafts. J Hepatol61: 1267-1275.
- Boteon Y.L, Laing R.W, Schlegel A, Wallace L, Smith A, Attard J,Bhogal R.H, Neil D.A, Hübscher S, Perera M.T.P (2018) CombinedHypothermic and Normothermic Machine Perfusion ImprovesFunctional Recovery of Extended Criteria Donor Livers. Liver
- Kron P, Schlegel A, de Rougemont O, Oberkofler C.E, Clavien P.-A,Dutkowski P (2016) Short Cool and Well Oxygenated - HOPE forKidney Transplantation in a Rodent Model. Ann Surg.
- Rouslin W, Ranganathan S (1983) Impaired Function of MitochondrialElectron Transfer Complex I in Canine Myocardial Ischemia: Loss ofFlavin Mononucleotide. J Mol Cell Cardiol.
- Stepanova A, Sosunov S, Niatsetskaya Z, Konrad C, Starkov A,Manfredi G, Wittig I, Ten V, Galkin A (2019) Redox-Dependent Lossof Flavin by Mitochondrial Complex I in Brain Ischemia/Reperfusion Antioxid Redox Signal 20: 608-622.
- Panconesi R, Flores Carvalho M, Mueller M, Meierhofer D,Dutkowski P, Muiesan P, Schlegel A (2021) Viability Assessmentin Liver Transplantation—What Is the Impact of Dynamic OrganPreservation? Biomedicines.
- Muller X, Schlegel A, Kron P, Eshmuminov D, Würdinger M, MeierhoferD, Clavien P, Dutkowski P (2019) Novel Real Time Prediction of LiverGraft Function during Hypothermic Oxygenated Machine PerfusionPrior to Liver Transplantation. Ann Surg 270: 783-790.
- Wang L, Thompson E, Bates L, Pither L, Hosgood S.A, Nicholson M.L,Watson C.J.E, Wilson C, Fisher A.J, Ali S (2020) Flavin Mononucleotideas a Biomarker of Organ Quality - A Pilot Study. Transplant Direct.
- Leemkuil M, Lier G, Engelse M.A, Ploeg R.J, de Koning E.J.P, T’HartA, Krikke C, Leuvenink H.G.D (2018) Hypothermic OxygenatedMachine Perfusion of the Human Donor Pancreas. Transplant Direct.
- Hamaoui K, Gowers S, Sandhu B, Vallant N, Cook T, Boutelle M,Casanova D, Papalois V (2018) Development of Pancreatic MachinePerfusion: Translational Steps from Porcine to Human Journalof Surgical Research 223: 263-274.
- Barlow A.D, Hamed M.O, Mallon D.H, Brais R.J, Gribble F.M, ScottA, Howat W.J, Bradley J.A, Bolton E.M, Pettigrew G.J (2015) Use ofEx Vivo Normothermic Perfusion for Quality Assessment of DiscardedHuman Donor Pancreases. Am J Transplant 15: 2475.
- Nassar A, Liu Q, Walsh M, Quintini C (2018) Normothermic Ex VivoPerfusion of Discarded Human Pancreas. Artif Organs 42: 334-335.
- Eshmuminov D, Becker D, Bautista Borrego L, Hefti M, SchulerJ, Hagedorn C, Muller X, Mueller M, Onder C, Graf R (2020) AnIntegrated Perfusion Machine Preserves Injured Human Livers for 1Week. Nat Biotechnol.
- Mueller M, Hefti M, Eshmuminov D, Schuler M.J, da Silva R.X.S,Petrowsky H, de Oliveira M.L, Oberkofler C.E, Hagedorn C, MancinaL (2021) Long-Term Normothermic Machine Preservation of PartialLivers: First Experience With 21 Human Hemi-Livers. Ann Surg 274:836-842.
- Nasralla D, Coussios C.C, Mergental H, Akhtar M.Z, Butler J, CeresaC.D.L, Chiocchia V, Dutton S.J, García-Valdecasas J.C, Heaton N(2018) A Randomized Trial of Normothermic Preservation in LiverTransplantation. Nature.
- Mergental H, Laing W, Kirkham A.J, Perera M.T.P.R, BoteonY.L, Attard J, Barton D, Curbishley S, Wilkhu M, Neil D.A.H (2020)Transplantation of Discarded Livers Following Viability Testing withNormothermic Machine Perfusion. Nat Commun 11.
- Gaurav R, Butler J, Kosmoliaptsis V, Mumford L, Fear C, SwiftL, Fedotovs A, Upponi S, Khwaja S, Richards J (2022) LiverTransplantation Outcomes From Controlled Circulatory Death Donors:SCS vs in Situ NRP vs Ex Situ NMP. Ann Surg 275.
- Kumar R, Chung Y, Runau F, Isherwood J.D, Kuan K.G, WestK, Garcea G, Dennison A.R (2018) Ex Vivo Normothermic PorcinePancreas: A Physiological Model for Preservation and TransplantStudy. Int J Surg 54: 206-215.
- Mazilescu I, Parmentier C, Kalimuthu S.N, Ganesh S, Kawamura M,Goto T, Noguchi Y, Selzner M, Reichman T.W (2022) Normothermic Ex Situ Pancreas Perfusion for the Preservation of Porcine PancreasGrafts. Am J Transplant.
- Wahlberg J, Southard H, Belzer F.O (1989) Preservation-Induced Pancreatitis in an Isolated Perfused Pancreas Model in theDog. Transpl Int 2: 165-167.
- Pegg E, Klempnauer J, Diaper M.P, Taylor M.J (1982) Assessmentof Hypothermic Preservation of the Pancreas in the Rat by aNormothermic Perfusion Assay. J Surg Res 33: 194-200.
- Eckhauser F, Knol J.A, Porter-Fink V, Lockery D, Edgcomb L,Strodel W.E, Webb D, Simmons J (1981) Ex Vivo NormothermicHemoperfusion of the Canine Pancreas: Applications and Limitationsof a Modified Experimental Preparation. J Surg Res 31: 22-37.
- Loubatières-Mariani M.M, Chapal J, Puech R, Lignon F, Valette G(1980) Different Effects of Hypothermia on Insulin and GlucagonSecretion from the Isolated Perfused Rat Pancreas. Diabetologia 18:329–333.
- O’Malley V.P, Keyes D.M, Postier R.G (1986) The Fluosol-PerfusedIsolated Canine Pancreas: A Model for the Study of Blood ComponentEffects in Acute Pancreatitis. J Surg Res 40: 210-215.
- Kenmochi T, Asano T, Nakagouri T, Enomoto K, Isono K, Horie H (1992)Prediction of Viability of Ischemically Damaged Canine PancreaticGrafts by Tissue Flow Rate with Machine Perfusion. Transplantation53: 745-750.
- Karcz M, Cook H.T, Sibbons P, Gray C, Dorling A, Papalois V (2010)An Ex-Vivo Model for Hypothermic Pulsatile Perfusion of PorcinePancreata: Hemodynamic and Morphologic Characteristics. Exp ClinTransplant 8: 55-60.
- Toledo Pereyra L.H, Valgee K.D, Castellanos J, Chee M (1980)Hypothermic Pulsatile Perfusion: Its Use in the Preservation ofPancreases for 24 to 48 Hours before Islet Cell ArchSurg 115: 95-98.
- Ogbemudia E, Hakim G, Dengu F, El-Gilani F, Dumbill R, Mulvey J,Sayal K, Prudhomme T, Mesnard B, Rozenberg K (2021) Developmentof Ex Situ Normothermic Reperfusion as an Innovative Method toAssess Pancreases after Preservation. Transpl Int 34: 1630-1642.
- Pravisani R, Baccarani U, Molinari E, Cherchi V, Bacchetti S, Terrosu G,Avital I, Ekser B, Adani L (2022) PO2 21% Oxygenated HypothermicMachine Perfusion in Kidney Transplantation: Any Clinical Benefit? IntJ Artif Organs 45: 666-671.
- Lazeyras F, Buhler L, Vallee J.P, Hergt M, Nastasi A, Ruttimann R,Morel P, Buchs B (2012) Detection of ATP by “in Line”31P MagneticResonance Spectroscopy during Oxygenated Hypothermic PulsatilePerfusion of Pigs’ Kidneys. Magnetic Resonance Materials in PhysicsBiology and Medicine 25: 391-399.
- Venema L.H, Brat A, Moers C, Hart N.A, Ploeg R.J, Hannaert P,Minor T, Leuvenink A.H.G.D (2019) Effects of Oxygen during Long-Term Hypothermic Machine Perfusion in a Porcine Model of KidneyDonation after Circulatory Death. Transplantation.
- Lindell S.L, Muir H, Brassil J, Mangino M.J (2013) HypothermicMachine Perfusion Preservation of the DCD Kidney: Machine J Transplant 2013: 1-7.
- Lindell S.L, Klahn S.L, Piazza T.M, Mangino M.J, Torrealba J.R,Southard J.H, Carey H. v (2005) Natural Resistance to Liver ColdIschemia-Reperfusion Injury Associated with the Hibernation Am J Physiol Gastrointest Liver Physiol 288: G473-80.
- Schlegel A, Kron P, Graf R, Clavien P.-A, Dutkowski P (2014)Hypothermic Oxygenated Perfusion (HOPE) Downregulates theImmune Response in a Rat Model of Liver Ann Surg260: 937-938.
- Dholakia S, Royston E, Sharples J, Sankaran V, Ploeg R.J, Friend P.J (2018) Preserving and Perfusing the Allograft Pancreas: PastPresent and Future. Transplant Rev 32: 127-131.
- Kuroda Y, Fujino Y, Kawamura T, Suzuki Y, Fujiwara H, Saitoh Y (1990)Mechanism of Oxygenation of Pancreas during Preservation by aTwo-Layer (Euro-Collins’ Solution/Perfluorochemical) Cold-Storage Transplantation 49: 694-696.
- Matsumoto S, Kandaswamy R, Sutherland D.E.R, Hassoun A.A,Hiraoka K, Sageshima J, Shibata S, Tanioka Y, Kuroda Y (2000)Clinical Application of the Two-Layer (University of Wisconsin Solution/Perfluorochemical plus O2) Method of Pancreas Preservation before Transplantation 70: 771-774.
- van Golen F, van Gulik T.M, Heger M (2012) Mechanistic Overview ofReactive Species-Induced Degradation of the Endothelial Glycocalyxduring Hepatic Ischemia/Reperfusion Injury. Free Radic Biol Med 52:1382-1402.
- Chance B, Thorell B (1959) Localization and Assay of RespiratoryEnzymes in Single Living Cells: Fluorescence Measurementsof Mitochondrial Pyridine Nucleotide in Aerobiosis and Nature.
- Liu Q, Simioni A, del Angel Diaz L, Quintini C (2020) PancreasPerfusion Preservation: State of the Art with Future Directions. ArtifOrgans 44: 445-448.
- Bellini M.I, Yiu J, Nozdrin M, Papalois V (2019) The Effect ofPreservation Temperature on Liver Kidney and Pancreas Tissue ATPin Animal and Preclinical Human Models. J Clin Med 8.
- Jomaa A, Gurusamy K, Siriwardana P.N, Claworthy I, Collier S, deMuylder P, Fuller B, Davidson B (2013) Does Hypothermic MachinePerfusion of Human Donor Livers Affect Risks of Sinusoidal EndothelialInjury and Microbial Infection? A Feasibility Study Assessing FlowParameters Sterility and Sinusoidal Endothelial Ultrastructure. InProceedings of the Transplantation Proceedings; 45: 1677-1681.
- Doppenberg B, Leemkuil M, Engelse M.A, Krikke C, de Koning E.J.P,Leuvenink H.G.D (2021) Hypothermic Oxygenated Machine Perfusionof the Human Pancreas for Clinical Islet Isolation: A ProspectiveFeasibility Study. Transpl Int 34: 1397-1407.
- Watson C, Kosmoliaptsis V, Pley C, Randle L, Fear C, Crick K,Gimson A, Alli M, Upponi S, Brais R (2018) Observations on the ExSitu Perfusion of Livers for Transplantation. American Journal ofTransplantation .
- Mohkam K, Nasralla D, Mergental H, Muller X, Butler A, JassemW, Imber C, Monbaliu D, Perera M.T.P, Laing R.W (2022) In SituNormothermic Regional Perfusion versus Ex Situ NormothermicMachine Perfusion in Liver Transplantation from Donation afterCirculatory Death. Liver Transpl.
- Stepanova A, Kahl A, Konrad C, Ten V, Starkov A.S, Galkin A(2017) Reverse Electron Transfer Results in a Loss of Flavin fromMitochondrial Complex I: Potential Mechanism for Brain IschemiaReperfusion Injury. Journal of Cerebral Blood Flow and Metabolism.
- Prudhomme T, Kervella D, Ogbemudia A.E, Gauttier V, le Bas‐Bernardet S, Minault D, Hervouet J, Cantarovich D, Karam G,Renaudin K (2020) Successful Pancreas Allotransplantations afterHypothermic Machine Perfusion in a Novel Diabetic Porcine Model: AControlled Study. Transplant International.
- Tanioka Y, Hering J, Sutherland D.E.R, Kronson J.W, KurodaY, Gilmore T.R, Aasheim T.C, Rusten M.C, Leone J.P (1997)Effect of Pancreatic Warm Ischemia on Islet Yield and Viability inDogs. Transplantation 64: 1637-1641.
- Corlett M.P, Scharp D.W (1988) The Effect of Pancreatic WarmIschemia on Islet Isolation in Rats and J Surg Res 45: 531-536.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.