The Individual Fluctuation Range and Significance of CA153 in Breast Cancer
Authors: Ning Wang*, Ping Chen, Jun Liu, Linping Huang, Meng Yang
*Corresponding Author: Ning Wang, Department of General Surgery, China-Japan Friendship Hospital, NO. 2, Yinghua East Street, Chaoyang District, Beijing, 100029, China
Received Date: 26 February, 2021
Accepted Date: 29 March, 2021
Published Date: 05 April, 2021
Citation: Wang N, Chen P, Liu J, Huang L, Yang M (2021) The Individual Fluctuation Range and Significance of CA153 in Breast Cancer. J Surg 6: 1380. DOI: https://doi.org/10.29011/2575-9760.001380
Abstract
Background: To observe the individual patient fluctuation range of carbohydrate antigen-153 in breast cancer and find the individual standard of carbohydrate antigen-153 to follow-up.
Methods: From January 2014 to December 2017, 138 patients with stage 0-2 breast cancer without metastasis were included and their carbohydrate antigen-153 value was compared to 113 patients with benign breast disease. Patients’ carbohydrate antigen-153 fluctuation was followed for 2-5 years to study their distribution range.
Results: The carbohydrate antigen-153 values both in benign breast diseases and stage 1-2 breast cancer was distributed in the normal range (0-25 U/ml). The peak value of the benign group and the cancer group was 7-8 U/ml and 10-11 U/ml, respectively. The mean value of 138 patients ranged between 3-30 U/ml. The average fluctuation range was 3.5 ± 2.6 U/ml. 90% of carbohydrate antigen-153 value is below 18 U/ml, and 90% of the fluctuation range is less than 6 U/ml or 50% of the average value. In patients with irregular level fluctuation, the peak was either at the first value (preoperative) or the second value (after surgery and chemotherapy), with different significance.
Conclusions: The distribution of carbohydrate antigen-153 value in stage 0-2 breast cancer patients was basically in the normal range. However, individual patient has specific carbohydrate antigen-153 fluctuation range. The increase of value higher than before, more than 3 U/ml or 50% of the average, indicates potential recurrence or metastasis thus close follow-up or further examination is required.
Keyword: Breast cancer; CA153; CA 15-3 Individual; Follow up
Background
Carbohydrate Antigen-153 (CA153) is a common marker of breast cancer. Due to its low sensitivity, it is generally used to monitor the prognosis instead of screening the breast cancer[1,2]. During follow-up, we found that CA153 of most early stage breast cancer patients kept at a low level and between a narrower range than its normal range. Therefore, whether CA153 follow up should be carried out in a more rigorous and individual manner, rather than the same standards remains to be explored.
Patients and Methods
The CA153 level from peripheral venous blood collected before breakfast was examined by chemo-luminescence immunoassay. The normal range was 0-25 U/ml, and the highest level can be assayed was 300 U/ml.
Step 1: From January 2014 to December 2017, the CA153 levels of 113 patients with benign breast disease and 138 patients with stage 0-2 breast cancer treated by our medical group were recorded and their distribution range was calculated. Inclusion criteria of benign breast disease were as follow: breast disease was confirmed to be benign by pathology; CA153 was measured preoperatively. Inclusion criteria of stage 0-2 breast cancer group were as follow: the breast cancer diagnosis was confirmed by pathological examination; stage 0-2; CA153 was measured preoperatively; ≥4 times of reexamination were performed; curative surgery followed by chemotherapy/endocrine therapy/radiotherapy/target therapy was performed routinely basing on athological result. The exclusion criteria were as follow: patients who had received neoadjuvant chemotherapy before the first measurement; recurrence and metastasis during surveillance.
Step 2: The 138 patients with stage 0-2 breast cancer were followed up for 4-19 times in 2-5 years. The median number of follow-up was 6 times. CA153 was measured originally on admission, and the second measurement was 6 months later after surgery and chemotherapy. The reexamination interval was 3-6 months.
The general conditions of the patients are shown in Table 1:
Step 3: Five cases which had representative fluctuations in each section were selected from 138 patients to represents the CA153 variation. Extraction criteria: more than 6 reexaminations were performed; their distributions were in different sections; Stable fluctuations.
Step 4: A total of 23 from 138 patients with irregular fluctuation were extracted for observation. Extraction criteria: one CA153 value higher than the adjacent value of more than 3 U/ml or the fluctuation range ≥ 50% of its own average value. Step 1 to step 4 are shown in Figure 1:
Data analysis was performed using Microsoft Excel. The measurement value was recorded as average standard deviation. u test was adopted for the approximate normal distribution and n >100.
Results
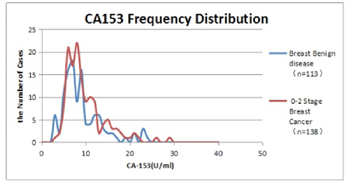
CA153 frequency distribution diagram of benign breast disease and stage 0-2 breast cancer is shown in Figure 2.
The distribution peak (7-8 U/ml) in patients with benign breast disease was slightly on the left, and the distribution peak (9-10 U/ml) in patients with stage 0-2 breast cancer was slightly on the right. However, most of the values were within the normal range (0-25 U/ml), and only a few in the breast cancer group were higher than normal (25 U/ml). The average value of benign group was 9.61 ± 4.56 U/ml, the average value of 0-2 stage breast cancer on admission was 10.70 ± 5.53 U/ml, u test, u=0.86 < 1.96, P > 0.05. CA153 is of little significance in differentiating the early stage malignancy from benign.
After multiple reexaminations, the average value of each patient’s average was 10.24 ± 4.49 U/ml, and the fluctuation range of each patient (the maximum - minimum value in each patient) was 3.5 ± 2.6 U/ml. The frequency distribution of the individual patient average value and fluctuation range in 138 patients were shown separately in Figure 3.
A is the case with the lowest CA153, E is the case with the highest and stable CA153, and C is the case with the most common CA153 distribution in the population. It can be seen that the value of each patient fluctuated within a relatively narrow range.
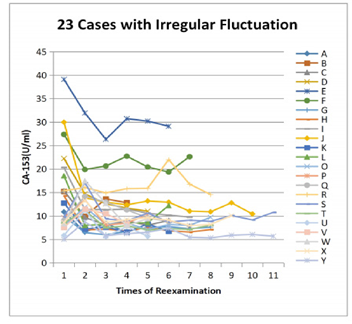
There were 23 patients with irregular fluctuation of CA153 value. The fluctuation ranges were more than 50% and there always was a single CA153 value higher than the adjacent one, more than 3U/ml. Among them, the highest value was identified in 12 patients on admission, and decreased after treatment, then remained stable. 10 patients had the highest value at the first reexamination in 6 months, which then returned to the original level at admission and remained stable. In the last case “R”, the baseline of CA153 was around 15 U/ml, with a fluctuation range of < 3 U/ml. At the 6th examination, CA153 elevated abnormally to 22.03 U/ml, close to the limit but normal, along with elevated CA125. After the diagnoses and treatment of ovary cancer, both CA153 and CA125 fell back to the baseline. The CA153 values of these 23 patients were plotted in Figure 5. Except the ovarian cancer patient, the other 22 patients’ general conditions are listed in Table 2.
Discussion
CA153 is a type of trans-membrane glycoproteins, which can be expressed apically in the near lumen or glandular surface of epithelial cells in various tissues and organs [1]. It was first found in breast cancer cells. When a cell turns malignant, the activation of glycosyl-transferase cause changes on the cell surface, CA153 is released from the cancer cells into the blood circulation [2]. Therefore, it can be applied as an indicator of breast cancer. However, preoperative levels of CA153 exhibits low diagnostic accuracy for early stage breast cancer [3]. The serum CA153 of patients with early breast cancer is rarely higher than the normal range, thus it cannot be used to diagnose breast cancer. CA153 is not recommended to be used for monitoring the efficacy of advanced breast cancer on its own [4], or as a routine variables of follow up without symptoms in some guideline [5]. Nevertheless, CA153 examination is still recommended prior to chemotherapy cycle since it is helpful to evaluate the efficacy of treatment, combined with CEA and imaging, especial for foci that could not be evaluated [6], and it may indicates recurrence and metastasis [7]. In our results, both CA153 of patients with stage 0-2 breast cancer and CA153 of patients with benign breast disease distributed within the normal range, only the peak of breast cancer curve lied slightly on the right of that of benign breast disease.
In addition to its role in follow-up monitoring, it has been found to be associated with the characteristics of breast cancer. For example, LI X [8] found that high CA153 level was associated with high pathological grade, younger age, and undoubtedly tumor burden as well. Meanwhile, elevated serum CA153 levels were also associated with other diseases. Li Xiulian [9] observed a total of 19,789 test results of serum CA153 levels from healthy individuals and patients with other types of diseases during 5 years, and found that patients with lung, breast, ovarian cancers, nephrotic syndrome, type 2 diabetes, endometrial cancer, coronary heart disease, cervical cancer, uremia, and other 12 diseases plus healthy people >65 years old had significantly increased median serum CA153 levels compared to that of healthy controls. So it was proposed that the increased serum CA153 levels might be associated with pathological leakage of the epithelial cell products into the blood circulation in addition to the decreased CA153 clearance rate.
All of the above shows that the CA153 level is related to a variety of factors. Due to the differences of individual conditions, everyone should have their own normal range of CA153. It was confirmed again in our study that CA153 of stage 0-2 breast cancer was still in the normal range (0-25 U/ml), therefore, CA153 had no practical significance for the diagnosis of early breast cancer. However, from the frequency distribution, it was found that the peak value of CA153 in breast cancer patients was on the right of that in patients with benign breast disease, suggesting that the value of CA153 in some early breast cancer patients might increase too, but still in the normal range. After continuous monitoring of CA153 in 138 patients, it can be seen that each patient had its own fluctuation range, and the range was narrow, 90% of them was below 6 U/ml. It indicates the existence of individual differences, 0-25 U/ml is only the normal range of the population, while the normal range of individual is different, which might be 5-7 U/ml, 10-13 U/ml, or 20-26 U/ml. The fluctuation range of each patient (the maximum - minimum value in each patient) was 3.5 2.6 U/ml, suggesting that each patient showed individual fluctuation range, which can be interpret as the individual patients normal range instead of 0-25 U/ml. 23 cases with individual fluctuation range more than 50% of the average value were extracted from the 138 patients.
It was found that CA153 value of 12 cases elevated abnormally on admission, then fell down and kept stable, which illustrated that early breast cancer also could release more CA153 to circulation, but it was difficult to be recognized due to the wider normal range of population. And the existence of CA153 increase on admission in some cases might explain the right shifting of breast cancer CA153 frequency distribution curve in Figure 2. Meanwhile, CA153 elevated after surgery and chemotherapy in 10 other cases, then decreased to the original level on admission and maintain stable, the highest value was obtained soon after surgery and chemotherapy. That suggested the possibility of transient CA153 release due to the destruction of systemic micrometastases after chemotherapy. Molina R [10] has also reported that serum markers may appear false elevation at the beginning of chemotherapy, which might share the same reason. It was interesting to find that cases with CA153 elevated soon after surgery and chemotherapy did not have positive Her-2. Due to the small amount, the phenomena would not be discussed any more here.
The CA153 value of the last case “R” was found increased to 22.03 U/ml combined with ca125 at the sixth reexamination, after ovarian cancer surgery, it fell back to the original level. This case suggests that CA153 can be used as an alert when it increases more than its individual range, even if it is still in the normal range of population. Knowing the individual patient fluctuation range of CA153, the clinical significance can be evaluated.
As a commonly used prognostic indicator for breast cancer, the increase of CA153 usually indicates metastasis.
It is necessary to obtain a patient’s normal range by multiple examinations during treatment and early follow up. After that, the patient would be re-examined every 3 to 6 months basing on her condition. Since an initial increase or a short-term increase of CA153 after treatment might happen, it is necessary that temporary higher value in that special period should be excluded. Furthermore, if an increase beyond the normal range of the individual patient occurs, it should be treated seriously. A comprehensive upgrade of the examinations (such as chest film upgrading to CT; adding a cranial scan; PET-CT, etc.) or close follow-up is required.
The shortcoming of this study is that benign breast disease patients instead of healthy people were regarded as control, for the possible influence of benign breast disease on CA153 level. That was limited by our patient group.
Usually, the CA153 value would continue to fluctuate within its individual range when no metastasis occur. If a metastasis appears, CA 15-3 tends to rise geometrically. Hence, the window period during which the CA153 value is higher than before but still within the normal range would be transient. If the followup interval is longer, it is likely that both significant increase of CA153 and visible metastasis on imaging are found at the same time. Only when the patient happens to be normal in a routine image examination, with the exception of a higher CA153 level than its individual range, the patient can be included in the earlywarning group. Though it is not clear whether all population has a window period, at least it may raise the doctor’s awareness. More metastatic cases study should be performed to observe the changing of CA153 combined with imaging changes. Since CA153 showed individual patients normal range, it is possible that other tumor markers have individual patients normal range as well which may improve the sensitivity of examination.
Conclusion
- It was confirmed that CA153 was of little significance in differentiating malignant from benign.
- The CA153 of each patient has its own baseline and range. The normal range of CA153 is suitable for the population, but wider than the normal range of an individual, which occupying a narrow section, 90% is less than 6 U/ml. 90% patients’ value is below 18 U/ml.
- If a single rise is greater than 3 U/ml, even when the value is less than 25 U/ml, it is necessary to be alert of the possibility of metastasis.
- Although the value of CA153 obtained preoperatively in some patients was normal, it was significantly higher than that measured after treatment, which was considered as tumor load. In some other patients, however, the CA153 obtained after surgery and chemotherapy were increasing significantly, then fell back to the original level as before, which might be explained by the possibility of CA153 released from destructed tumor cells after chemotherapy.
- Therefore, CA153 baseline and fluctuation range of the individual patients should be obtained by multiple examinations in each breast cancer patient. Further, the CA153 could be monitored according to the individual normal range rather than the overall normal range of the population. Both preoperative and post-treated abnormalities might be present temporarily in some patients.
Figures


Tables


References
- Dnistrian AM, Schwartz MK, Greenberg EJ, et al. (1991) CA 15-3 and carcinoembryonic antigen in the clinical evaluation of breast cancer. Clin Chim Acta 200: 81-93.
- Mariani L, Miceli R, Michilin S, et al. (2009) Serial determination of CEA and CA 15.3 in breast cancer follow-up: an assessment of their diagnostic accuracy for the detection of tumour recurrences. Biomarkers 14: 130-136.
- Lian M, Zhang C, Zhang D, et al. (2019) The association of five preoperative serum tumor markers and pathological features in patients with breast cancer. J Clin Lab Anal 33: e22875.
- Gradishar WJ, Anderson BO, Balassanian R, et al. (2016) Invasive Breast Cancer Version 1.2016, NCCN Clinical Practice Guidelines in J Natl Compr Canc Netw 14: 324-354.
- Khatcheressian JL, Hurley P, Bantug E, et al. (2013) Breast cancer followup and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 31: 961-965.
- Molina R, Barak V, van Dalen A, et al. (2005) Tumor markers in breast cancer- European Group on Tumor Markers recommendations. Tumour Biol 26: 281-293.
- Surgeon C (2002) Practice Guidelines for Tumor Marker Use in the Clin Chen 48: 1151.
- Li X, Dai D, Chen B,et al. (2018) Clinicopathological and Prognostic Significance of Cancer Antigen 15-3 and Carcinoembryonic Antigen in Breast Cancer: A Meta-Analysis including 12,993 Patients. Dis Markers 2018.
- Li X, Xu Y, Zhang L (2019) Serum CA153 as biomarker for cancer and noncancer diseases. Prog Mol Biol Transl Sci 162: 265-276.
- Molina R, Auge JM, Farrus B, et al. (2010) Prospective evaluation of carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 15.3 in patients with primary locoregional breast cancer. Clin Chem 56: 1148-1157.

 research article
research article